

Ready-To-Use Media Plates for environmental monitoring of air, surfaces and personnel and assurance of reproducible detection of microorganisms in most critical areas like isolators and clean room in Pharmaceutical industries.
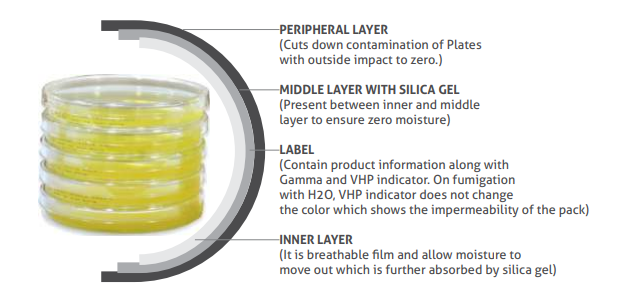
The plates are irradiated and triple-wrapped in transparent packaging. Includes the permeable film, Gamma & VHP indicator on the descriptive label. Details such as expiry date, product name, code, batch no. are printed on the edge of the plate. The user has an advantage of clarity while reading colony on colony counter.
Absolute EM is quality controlled in accordance with BP/EP/JP/USP/IP
Certificate of Analysis (COA), Stability Data, Instructions For Use (IFU), Transportation Study, Gamma Irradiation Certificate, Packing Integrity Test Report, VHP Impermeability Certificate, Microbial Validation.
The plates are available in 90mm for passive and active air sampling applications and 55mm contact plates for microbiology testing of surfaces and personnel.